Cannabidiol (CBD)
Cannabidiol (CBD) is a naturally occurring non-psychotropic cannabinoid of the hemp plant Cannabis sativa L. and has been proven to induce several physiological and pharmacological effects. While CBD is approved as a medicinal product subject to prescription, it is also widely sold over the counter (OTC) in different forms.
Regarding the consumer safety of these OTC products, the question whether or not CBD might be converted into psychotropic cannabinoids, most prominently tetrahydrocannabinol (THC), under in vivo conditions, especially acidic condition of stomach, has initiated an ongoing scientific debate.
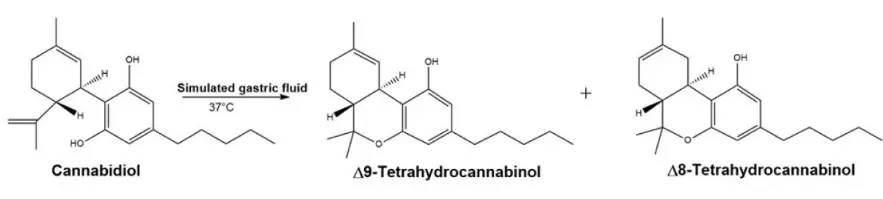
Conversion of CBD to the psychotropic forms ∆9-THC and ∆8-THC upon treatment with strong acids, such as HCl, sulfuric acid or p-toluenesulfonic acid, is doubtlessly proved by many publications. Some of these findings were demonstrated to also occur under in vivo conditions, e.g., by using simulated gastric juice for incubation. However, the transfer of these results to in vivo conditions seems to be the major point of the ongoing controversy as the in vivo conversion of CBD to ∆9-THC was not supported by all the animal studies.
GelEntroCeutics Enteric Softgel formulation for CBD will end to this controversy.
Enteric CBD will pass through the stomach, after it is swallowed, without releasing its content into acidic gastric juice and it will deliver CBD directly into intestine. Therefore, neither manufacturer, nor the consumer need to worry about the conversion of CBD to THC.
