Bioequivalence Study Consultation
A generic pharmaceutical product that has been approved can be used as an alternative of the innovator (reference) drug product.
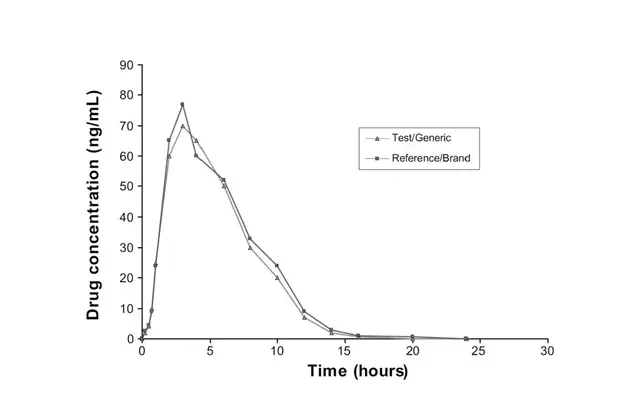
A Bioequivalence study is frequently designed after employing a double blind, crossover design to establish comparison among healthy volunteers. Bioequivalence may then be assessed using reliable statistical methods while taking into account the pre-specified regulatory requirements for bioequivalence.
Bioequivalence assessment is one of the most controversial problems, as bioequivalence does not always indicate therapeutic equivalent, and approval of generic does not ensure bioequivalence.
There are four factors to consider, when assessing the bioequivalence for generic medication approval:
- Although the absorption characteristics of the drugs are not identical, they are therapeutically comparable.
- Although the patterns of drug absorption are comparable, they are not therapeutically identical
- The absorption characteristics of generic and reference drugs are not the same, and they have different equivalency.
- Despite the similarity in medication absorption patters, they may not be therapeutically comparable.
Bioequivalence documents are the evidences that a generic product is bioequivalent with the innovator product which comprise a set of stage:
Subject selection
Wash out
Blood sampling
Statistical methods
GelEntroCeutics experts will help you to design the most suitable bioequivalence study for your target softgel products.
——- GET IN TOUCH ——-
